Smallest Piece Of An Element
- Slides: 34
Download presentation

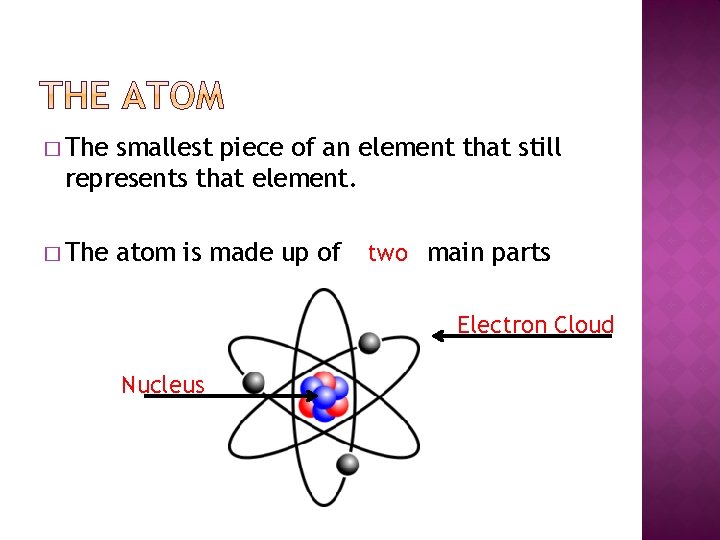
� The smallest piece of an element that withal represents that element. � The atom is fabricated upwardly of two main parts Electron Cloud Nucleus

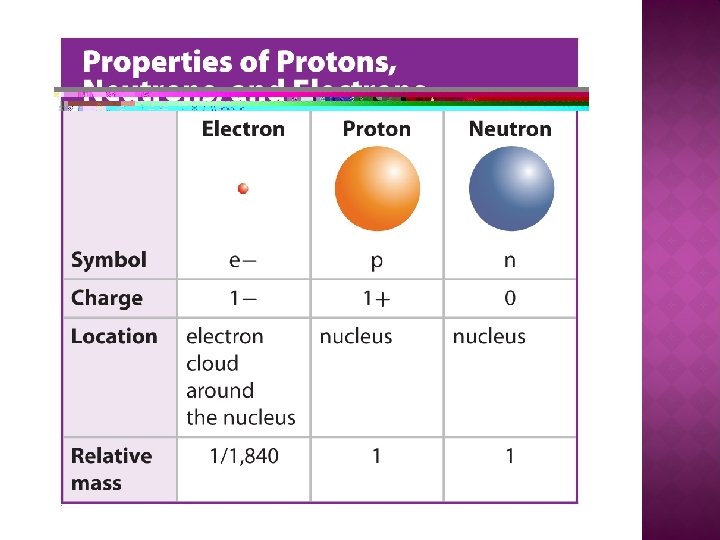
�A small expanse in the center of an atom �Responsible for near of the atom'due south mass �Contains positive charge � Contained in the nucleus: protons neutrons
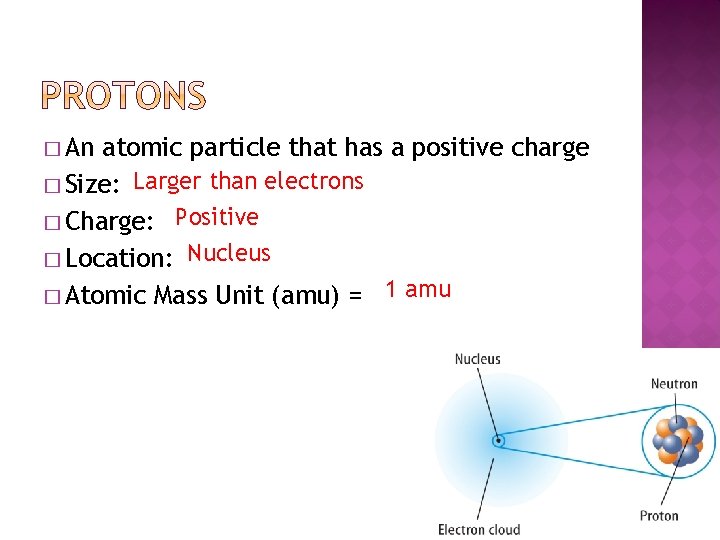
� An atomic particle that has a positive accuse � Size: Larger than electrons � Accuse: Positive � Location: Nucleus � Atomic Mass Unit (amu) = i amu
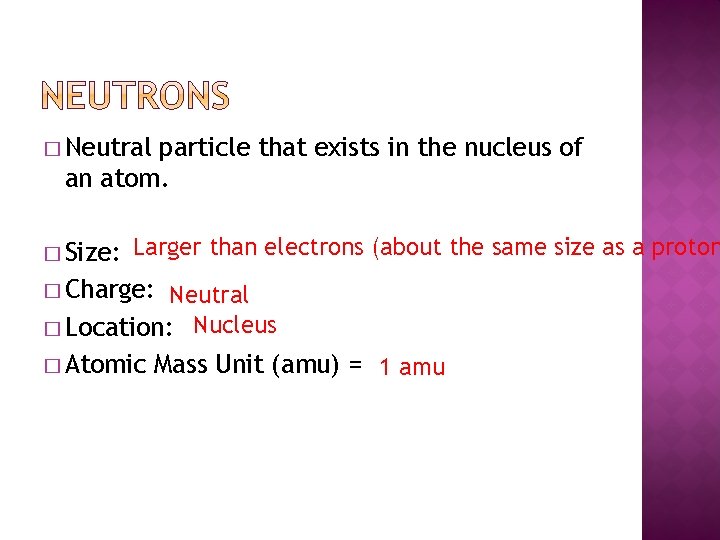
� Neutral particle that exists in the nucleus of an cantlet. � Size: Larger than electrons (about the aforementioned size as a proton � Charge: Neutral � Location: Nucleus � Atomic Mass Unit of measurement (amu) = 1 amu

� The area around an diminutive nucleus where an electron is about probable to be located � Contained in the electron deject: electrons
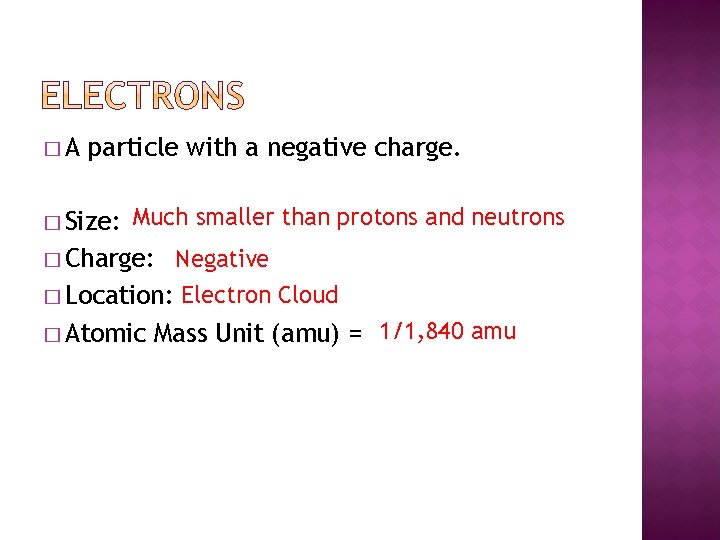
�A particle with a negative charge. � Size: Much smaller than protons and neutrons � Accuse: Negative � Location: Electron Cloud � Diminutive Mass Unit of measurement (amu) = i/1, 840 amu

� Democritus � Aristotle � John Dalton � J. J. Thomson � Ernest Rutherford � James Chadwick � Niels Bohr

� Proposed that different types of matter are fabricated from different types of atoms. � Proposed that space betwixt atoms was "empty"

� 384 – 322 B. C. � Did not believe in Democritus' idea that between atom's were empty space � He believed all matter was fabricated of burn, water, air and globe.

� Late 1700'south � All matter is made of atoms that cannot exist created, divided or destroyed. � Atoms of the same chemical element have the aforementioned mass and are exactly alike

� 1897 � Found negatively charged particles (electrons) and reasoned that they must have a positive charge that balances them out.

� Thomson'due south student � Kickoff atomic model with nucleus � Came up with the word proton for positive charge

� Discovered the neutron � Completed the atomic model
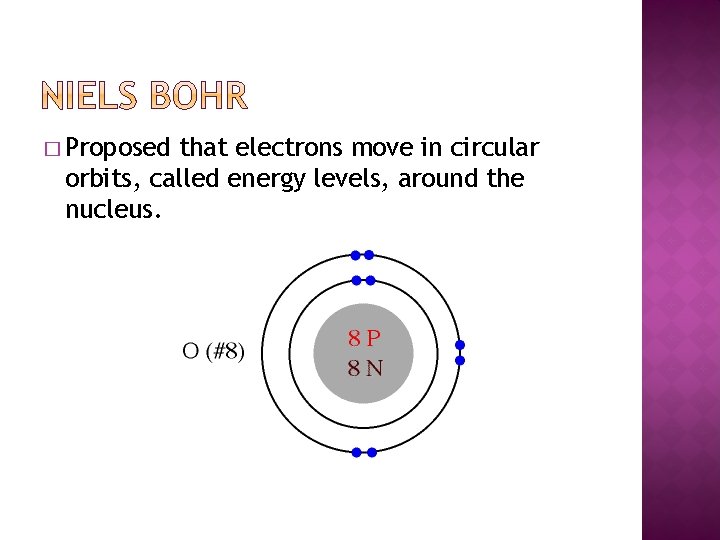
� Proposed that electrons move in circular orbits, called energy levels, effectually the nucleus.

� Pure substance fabricated of only i type of atom � A substance made from atoms that all take the same number of protons. In the empty box in your notes, look at the periodic table and fill out an chemical element box for carbon
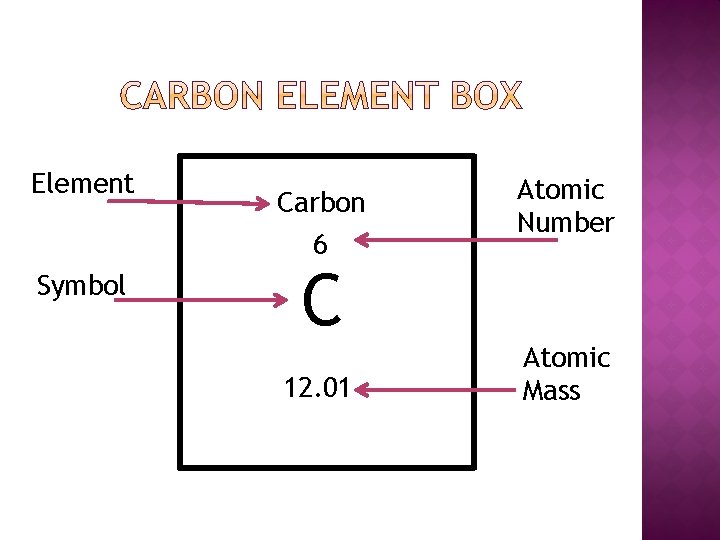
Element Symbol Carbon half dozen C 12. 01 Atomic Number Diminutive Mass

� The number of protons located in the cantlet's nucleus. � Look at the periodic table …can you find the atomic number in the post-obit chemical element boxes? Oxygen Potassium Gilt
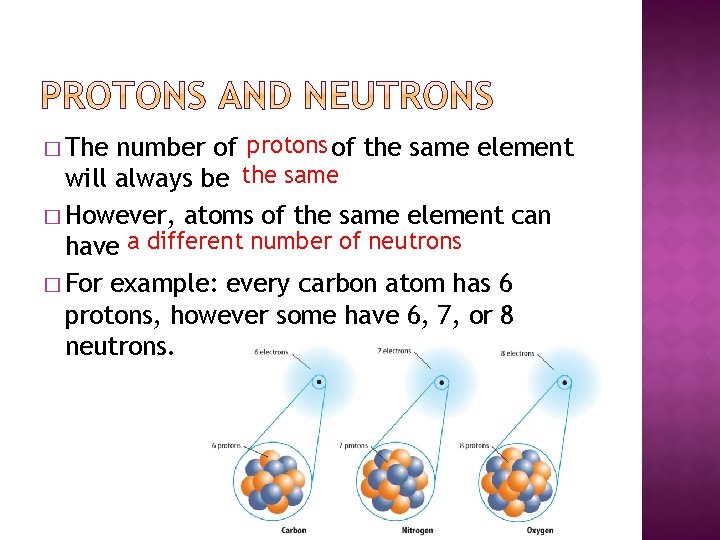
number of protons of the same element will e'er exist the same � However, atoms of the same element can accept a different number of neutrons � For case: every carbon atom has 6 protons, yet some have 6, vii, or 8 neutrons. � The

� Isotopes - atoms of the same chemical element that have different numbers of neutrons. � Virtually elements take several isotopes.
� The sum of protons and neutrons in an atom. average mass of the element's isotopes, weighted according to the abundance of each isotope.
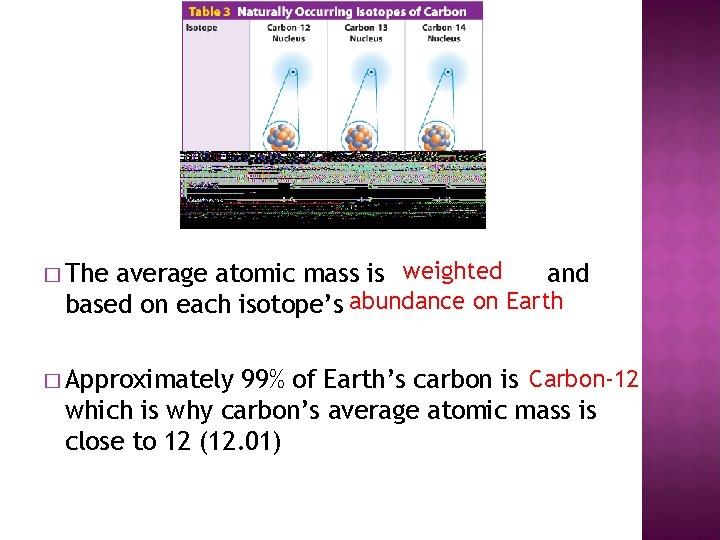
average atomic mass is weighted and based on each isotope's abundance on Earth � The � Approximately 99% of World's carbon is Carbon-12 which is why carbon's boilerplate atomic mass is close to 12 (12. 01)
� Bundled by: Dmitri Mendeleev (1834 -1907) � Bundled in order of: Increasing atomic mass � Periodic- Describes something that occurs or repeats in regular intervals.
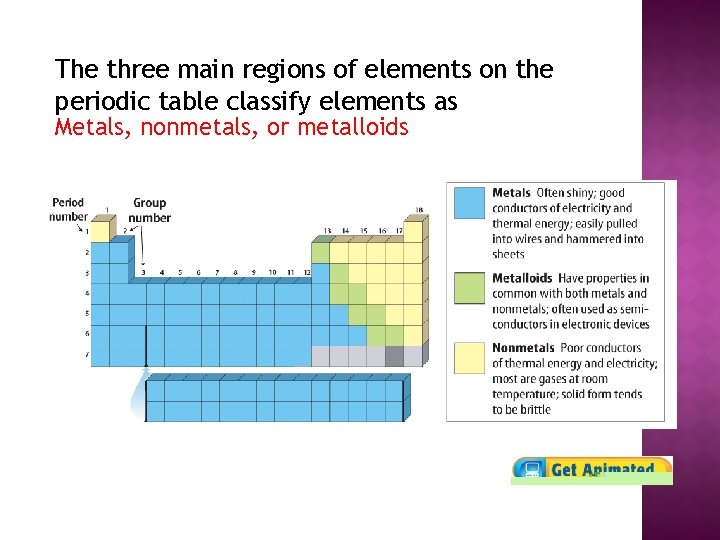
The three main regions of elements on the periodic table classify elements equally Metals, nonmetals, or metalloids

� The vertical (upward and down) columns of the periodic table are called groups or families � At that place are 18 groups � Elements in the aforementioned grouping or family have Similar chemic and physical backdrop

� The horizontal rows of the periodic table are called periods � Elements in a menstruation are not alike in properties � The first element in a period is normally an agile solid , and the concluding chemical element in a flow is always an inactive gas.

� Atomic number (number of protons) increases from left to right across a flow � Diminutive mass (number of protons + neutrons) increases from left to right across a catamenia � Metals are on the left � Non-metals are on the right
� Different electrons inside an atom accept unlike amounts of energy � An electron moves effectually the nucleus at a distance that corresponds to the amount of energy information technology has. � Electrons closest to the nucleus have the to the lowest degree corporeality of energy. � Electrons furthest from the nucleus have the most energy.
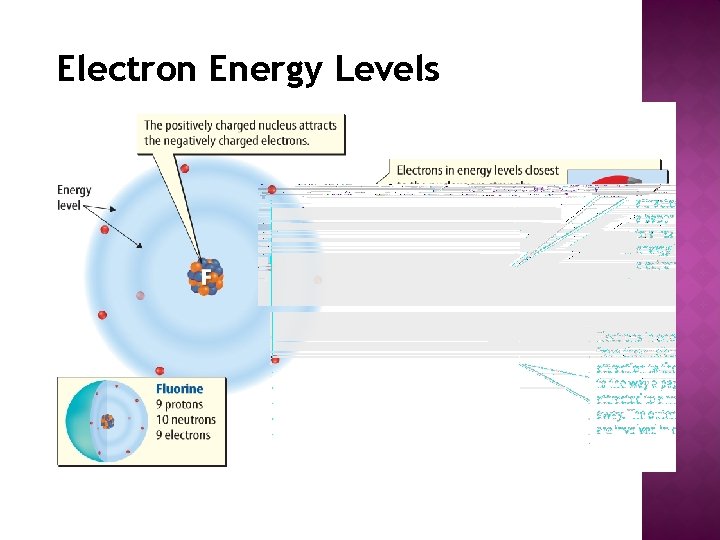
Electron Energy Levels

charged electrons are strongly attracted to positively charged nucleus of an atom. � Negatively � These OUTERMOST electrons can easily be attracted to the nucleus of other atoms � We call these OUTERMOST electrons valence electrons
� Valence Electrons - outermost electron of an atom that participates in chemical bonding. � These attractions are what cause chemic bonds

number of valence electrons in each cantlet of an element can assist make up one's mind � The type and the number of bonds information technology can form � The exception to finding number of valence electrons is helium
1 1 valence electron � Group 2 two valence electron � Group three -12 valence electrons vary � Grouping 13 three valence electrons � Group 14 4 valence electrons � Group 15 5 valence electrons � Group xvi six valence electrons � Group 17 7 valence electrons � Group 18 viii valence electrons � Helium is the exception and has 2 5. E. � Group **go back to the periodic table in your notes and label the valence electrons.
Smallest Piece Of An Element,
Source: https://slidetodoc.com/the-smallest-piece-of-an-element-that-still/
Posted by: owenssyclee.blogspot.com


0 Response to "Smallest Piece Of An Element"
Post a Comment